Abstract
Background: Older patients with acute myeloid leukemia (AML) who are ineligible for intensive chemotherapy are unlikely to achieve remission with available therapy and have unacceptably short survival. Venetoclax (VEN) is a small molecule inhibitor of BCL-2 that achieved remission rates of >60% combined with low-dose cytarabine (LDAC). Presented are long-term outcomes, including 1-year overall survival (OS) and biomarker analyses.
Methods: This phase 1b/2, open-label study (NCT02287233) evaluates the safety and preliminary efficacy of orally administered VEN combined with LDAC in patients ≥65 years with previously untreated AML (except for hydroxyurea). Patients were ineligible for intensive chemotherapy because of comorbidity or other factors and had an ECOG performance score of 0-2, with adequate hepatic and renal function. Exclusion criteria were acute promyelocytic leukemia, active CNS involvement with AML, concominant use of moderate or strong CYP3A inhibitors, or prior treament with cytarabine for a preexisting myeloid disorder. Prior treatment for myelodysplastic syndrome (MDS) was allowed. In cycle 1, VEN was started at 50 mg/day PO and increased over a 5-day ramp-up to reach the designated cohort dose of 600 or 800 mg/day on day 6, which was continued through day 28. In subsequent cycles, the desingated dose of VEN 600 or 800 mg/day was administered on days 1-28. LDAC 20 mg/m2/day SQ was given on days 1-10 of each cycle. Preliminary efficacy was assessed as the overall response rate (ORR, which included complete remission [CR], CR with incomplete blood count recovery [CRi], and partial remission [PR]). Adverse events (AEs) and laboratory values were monitored. Exploratory analysis of biomarkers (eg, cytogenetics, molecular markers) was performed to identify potential predictors of clinical outcomes.
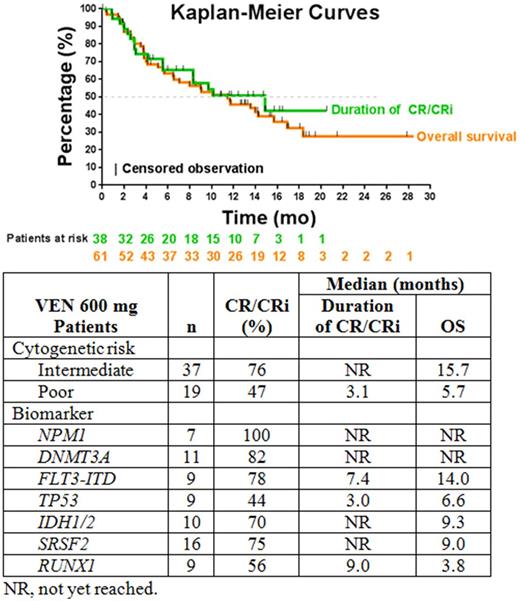
Results: Data cutoff was May 30, 2017. All 71 patients were enrolled ≥1 year prior (46 [65%] male; median age, 74 years [range, 66-87 years]): 10 received VEN 800 mg and 61 received VEN 600 mg, the recommended phase 2 dose. Thirty-three patients (47%) had a history of antecedent hematologic disorder (AHD), most commonly MDS. Among 61 patients given VEN 600 mg, median time on VEN treatment was 6 months (range, <1 to 21 months). Thirty-eight (62%) of these patients achieved CR/CRi with a median duration of CR/CRi of 14.9 months (95% CI, 5.6 months to not reached [NR]; Figure). Best responses were 26% CR, 36% CRi, and 2% PR. Median OS was 11.4 months (95% CI, 5.7-15.7 months); the observed 12-month OS was 46% (95% CI, 33-58%). Only 1 patient has subsequently undergone bone marrow transplantation. Treatment-emergent grade 3/4 AEs (in ≥20% of 61 patients) were thrombocytopenia (59%), neutropenia (46%), febrile neutropenia (36%), anemia (28%), and decreased WBC count (26%). One case (2%) of tumor lysis syndrome occurred. Serious AEs (in ≥3 of 61 patients) were febrile neutropenia (20%), malignant neoplasm progression (13%), lung infection/pneumonia (13%), and sepsis (7%). The 30-day mortality rate was 3%; causes of death were disease progression (n=1) and lung infection (n=1). Common recurrent mutations in 53 patients who received VEN 600 mg are shown in the Table. All patients with an NPM1 mutation (including 3 with a co-mutation in FLT3-ITD) achieved CR/CRi. Patients with DNMT3A, FLT3-ITD, and SRSF2 mutations had CR/CRi rates of ≥75%, whereas those with TP53 mutations had the lowest CR/CRi rates of 44%. For patients with CR/CRi, median OS was 18.4 months (95% CI, 13.5 months to NR). The 12-month OS rate for patients in the 600-mg VEN cohort who achieved CR/CRi was 70.4% from Kaplan-Meier estimates, with 11 deaths. Among 19 patients who received study treatment ≥12 months, 17 remain alive. The longest, ongoing, disease-free follow-up after treatment completion is 12 months.
Conclusions: The safety profile of VEN 600 mg/day plus LDAC was acceptable for elderly patients with treatment-naive AML who were ineligible for intensive chemotherapy. After ≥1 year of follow-up, the observed median OS was 11.4 months. This cohort included 44% (27/61) of patients with AHDs. Corelations of specified AML mutations with response and duration should be confirmed in later trials. Due to the observced CR/CRi rate of 62%, extended duration of response, and encouraging OS in a cohort of patients with particularly poor-risk features, the 600-mg dose of VEN combined with LDAC is being tested in an ongoing phase 3 study.
Wei: AbbVie, Celgene, Novartis, Amgen, Servier: Honoraria; AbbVie, Celgene, Servier: Research Funding; AbbVie, Celgene, Novartis, Amgen, Servier: Membership on an entity's Board of Directors or advisory committees. Strickland: Boehringer-Ingelheim: Consultancy, Research Funding; Sunesis: Consultancy, Research Funding; Novartis: Consultancy; Tolero: Consultancy; Astellas: Consultancy; CTI BioPharma: Consultancy; Baxalta: Consultancy. Roboz: AbbVie, Agios, Amgen, Amphivena, Array Biopharma Inc., Astex, AstraZeneca, Celator, Celgene, Clovis Oncology, CTI BioPharma, Genoptix, Immune Pharmaceuticals, Janssen Pharmaceuticals, Juno, MedImmune, MEI Pharma, Novartis, Onconova, Pfizer, Roche Pharmace: Consultancy; Cellectis: Research Funding. Hou: Teva Oncology, Seattle Genetics: Speakers Bureau. Fiedler: Amgen, Pfizer: Research Funding; Amgen, Gilead, GSO, Teva, Jazz Pharmaceuticals: Other: Support for meeting attendance; Amgen: Patents & Royalties; Amgen, ARIAD/Incyte: Membership on an entity's Board of Directors or advisory committees. Lin: Jazz Pharmaceuticals: Consultancy. Walter: ADC Therapeutics: Research Funding; Aptevo Therapeutics: Research Funding. Chyla: Abbvie: Employment, Equity Ownership. Popovic: AbbVie: Employment, Equity Ownership. Fakouhi: AbbVie: Employment, Equity Ownership. Shah: AbbVie: Employment, Equity Ownership. Dunbar: AbbVie: Employment, Equity Ownership. Xu: AbbVie: Employment, Equity Ownership. Mabry: AbbVie: Employment, Equity Ownership. Hayslip: AbbVie: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.